Raman spectroscopy is a widespread analytical technique used to study vibrational, rotational and other low-frequency states of molecules and consists in measuring the inelastic scattering of radiation. In traditional Raman spectroscopy and microscopy, visible (455, 532, 633 nm, etc.) or near-infrared radiation (most commonly 1064 nm) in the form of a focused laser beam is commonly used to excite molecules.

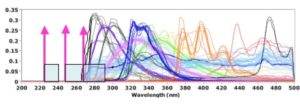
UV Raman spectroscopy uses ultraviolet lasers with a wavelength below 400 nanometers for excitation. In practice, the term far (or deep) UV Raman spectroscopy is also often used if the excitation wavelength is less than 280 nm (Figure 1).
In the past, the wider spread of this alternative to classical Raman spectroscopy was hindered mainly by the price and limited selection of the necessary excitation UV lasers and the generally more expensive construction of such a spectrometer. Due to the expanding possibilities of modern detectors, spectrographs and lasers, however, all obstacles are gradually being removed for a more general and more frequent use of this very promising variant of Raman spectroscopy.
Advantages of UV Raman spectroscopy and microscopy:
- Increased sensitivity: The use of UV excitation can increase the sensitivity of Raman spectroscopy and the intensity (signal to noise ratio) of the obtained Raman spectra. This is particularly useful for studying materials that have weak Raman spectra when excited at a longer wavelength (e.g. 532 nm). Raman scattering is in principle an extremely weak phenomenon. After irradiating the sample with an excitation laser, only about one photon out of 106 – 108 photons is detected as Raman radiation. UV excitation can help quite significantly here, because theoretically there is a relationship between the intensity of Raman scattering and the wavelength of the excitation laser, i.e. the intensity of light scattered by a molecule is inversely proportional to the fourth power of its wavelength. It follows that, for example, a 248 nm excitation laser can generate approximately 100x stronger signal than a 785 nm laser.
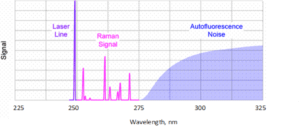
- Elimination of fluorescence: Fluorescence is a phenomenon that often competes with Raman radiation and can overlap the entire Raman spectrum. The fluorescence response of samples tends to be greatest when their molecular structure is complex (organic compounds and biological samples), but can also occur due to fluorescent impurities. It can be reduced by using a longer wavelength such as 785 nm or 830 nm, and is often completely eliminated by using 1064 nm excitation. Another option is to excite Raman spectra at wavelengths below the so-called fluorescence window precisely by means of UV excitation. Fluorescence typically occurs at wavelengths longer than 300 nm, so when using an excitation laser below about 300 nm, the entire usual spectral range can be easily obtained without its influence (Figure 2). The Raman spectrum and fluorescence of the sample can thus be measured as two separate phenomena that do not influence each other much. At UV wavelengths, the fluorescence and Raman spectrum are separated. UV Raman spectroscopic analysis thus enables the study of the structure and dynamics of complex materials, such as proteins and nucleic acids. In addition, using an excitation source in the UV region can lead to a relatively large (up to 2-4 orders of magnitude) resonance amplification of the Raman signal in some samples, a measurement technique in practice called UV-Resonance Raman (UVRR).
- Higher spatial resolution: UV radiation has a shorter wavelength than visible or infrared radiation, which allows for better spatial resolution when examining molecular structures using techniques such as mapping or sample imaging.
Main current applications of UV Raman spectroscopy:
- Biopharmacy and biomedicine (virological analysis, detection of yeast, pollen or microbial contamination, biological imaging, detection of bacterial cells and spores, etc.).
- Process on-line/at-line analysis (especially pharmaceutical production and food industry).
- Defense and security forces (detection of explosives and their precursors, identification of dangerous materials, forensic palynology, detection of narcotics, analysis of forgeries, crime scene investigation, etc.).
- Semiconductor materials manufacturing and research (“bandgap” semiconductor photoluminescence testing, semiconductor failure analysis, etc.)
- Analysis of water purity in the environment (chemical and biological contamination, quantitative analysis of nitrates and nitrides, etc.)
Nicolet CZ offers UV Raman (or deep UV Raman) spectrometers and microscopes in the form of highly scientific, custom-built systems from the German company S&I Spectroscopy & Imaging GmbH or UV excitation directly dedicated laboratory, operational and hand-held systems from the American company Photon Systems.
If you have any questions, don’t hesitate to contact us.
