Protein analysis by methods of molecular spectroscopy
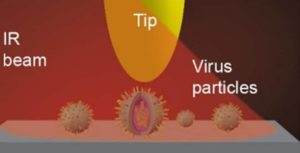 Proteins are the cornerstones of all life on Earth. They can take an unimaginable number of shapes and perform a wide range of functions, from the transcription of DNA in the nucleus to the destruction of foreign objects in the cell. Using vibrational spectroscopy, we can monitor the conformation of proteins and enzymes, their changes or the kinetics of enzymatic reactions. Unlike other methods, it is usually not necessary to modify the sample in any way to measure vibrational spectra, so the molecules can be monitored in their natural environment.
Proteins are the cornerstones of all life on Earth. They can take an unimaginable number of shapes and perform a wide range of functions, from the transcription of DNA in the nucleus to the destruction of foreign objects in the cell. Using vibrational spectroscopy, we can monitor the conformation of proteins and enzymes, their changes or the kinetics of enzymatic reactions. Unlike other methods, it is usually not necessary to modify the sample in any way to measure vibrational spectra, so the molecules can be monitored in their natural environment.
- Application description
- Raman spectroscopy
- FT-IR spectroscopy
Thanks to specialized accessories (tempered transmission cuvettes) and dedicated software (protein interpretation and analysis, database of known protein structures, deconvolution, prediction of secondary structures, automated buffer subtracting etc.), all FT-IR spectrometers we offer can be modified in order to make these measurements even easier: to better understand the fascinating world of proteins and enzymes.
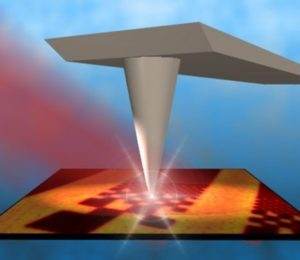 Another option is to use Neaspec‘s highly scientific spectrometers to analyze the secondary structure of individual protein complexes using nano-FTIR spectrometers that combine near-field microscopy (s-SNOM) technology and FT-IR spectroscopy. This technology can analyze proteins with a spatial resolution of less than 30 nanometers.
Another option is to use Neaspec‘s highly scientific spectrometers to analyze the secondary structure of individual protein complexes using nano-FTIR spectrometers that combine near-field microscopy (s-SNOM) technology and FT-IR spectroscopy. This technology can analyze proteins with a spatial resolution of less than 30 nanometers.