Researchers from the University of Georgia and Georgia State University used a neaSNOM microscope to study the mechanism of penetration of individual so-called enveloped viruses to host cell membrane in nanoscale. Different types of viruses can enter a host cell differently. The most common penetration process is the complex fusion of the virus into the cell membrane. In this experiment, the researchers performed a direct quantitative investigation of this fusion process at the vir-cell membrane interface. Measurements were performed at the size of a single virus using nano-FTIR spectroscopy and imaging. Comprehensive studies of the behavior of viruses penetrating the cell membrane provide vital information for the development of antiviral therapies and especially vaccines against many infections affecting humanity: influenza, HIV, Ebola and, of course, the new coronavirus. This study was recently published here in PLOS ONE.
So called enveloped viruses have their genome surrounded by a phospho-lipid envelope (membrane) as they move between host cells. Penetration of the cell membrane by the enveloped virus is a key step in the process of cell infection. In particular, it is important to understand how the enveloped virus interacts with host cell receptors and what structural changes occur in the envelope itself during membrane fusion. Numerous studies have established a recognized model of the fusion mechanism between the target and viral membranes. This model assumes that pores can only be formed when the target and viral membranes are subjected to membrane fusion to mediate vir-cell penetration. However, recent observations indicate that target and viral membranes rupture prior to this fusion. In addition, studies of adenoviral proteins and host cells have shown that host cell membranes can be destroyed without virus fusion after virus entry. On the other hand, the viral envelope and membrane of the target host cell have different chemical compositions and structure. The requirements for the formation of pores in each membrane are therefore different, so that the target rupture of the host or viral membrane can also be induced independently. In short, the mechanism of penetration and membrane behavior is still discussed. The reason is the high difficulty of direct observation of these phenomena at the nanoscale and especially the need for chemically specific imaging of this process. Clarification of the complex mechanism of collective fusion between a single virus and a host cell may provide useful information for the design of antiviral compounds.
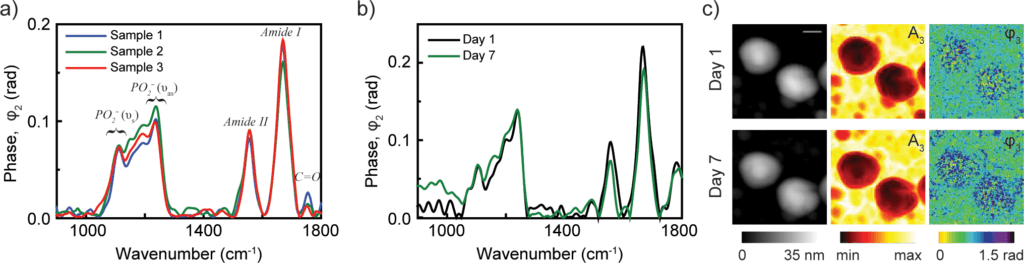
a) nano-FTIR spectra of influenza virus particles from three different samples at neutral pH (reproducibility of nanoparticle analysis)
b) Spectra from virus particles collected on day 1 and day 7 which show the stability of the virus particles
c) Images of topography, reflectance and absorption of two influenza virus particles on days 1 and 7
In principle, changes in the chemical and structural composition of the membranes of viral and host cells caused by the investigated process of viral infection at the molecular level can be detected by infrared spectroscopy. However, the characteristic size of viruses, lipid envelopes, and surface glycoproteins mediating the fusion process is much smaller than the diffraction limit for infrared light, which prevents the study of the behavior of individual “players” of infection. Therefore, it is important to find a tool that can provide spatial resolution at the nanoscale while detecting mechanical and chemical properties (e.g. the absorption of molecule-specific infrared radiation). Researchers Yohannes Abate and Ming Luoused spectroscopic infrared nano-imaging provided by the neaSNOM microscope to study the chemical and structural changes that occur prior to membrane fusion in a single archetypal influenza virus X31 at different pH environments. With fully quantitative data provided by the application of the nano-FTIR methodology, it was also possible to quantitatively evaluate the efficacy of an antiviral compound (Compound 136) in preventing viral membrane disruption: a new mechanism for inhibiting virus entry into the cell. Neaspec’s microscopes with IR nanoimaging and nano-FTIR spectroscopy provide a unique tool for analyzing the mechanisms underlying the functionality of viral and cell membranes at the nanoscale, which can significantly support advances in basic virus research and treatment development.
